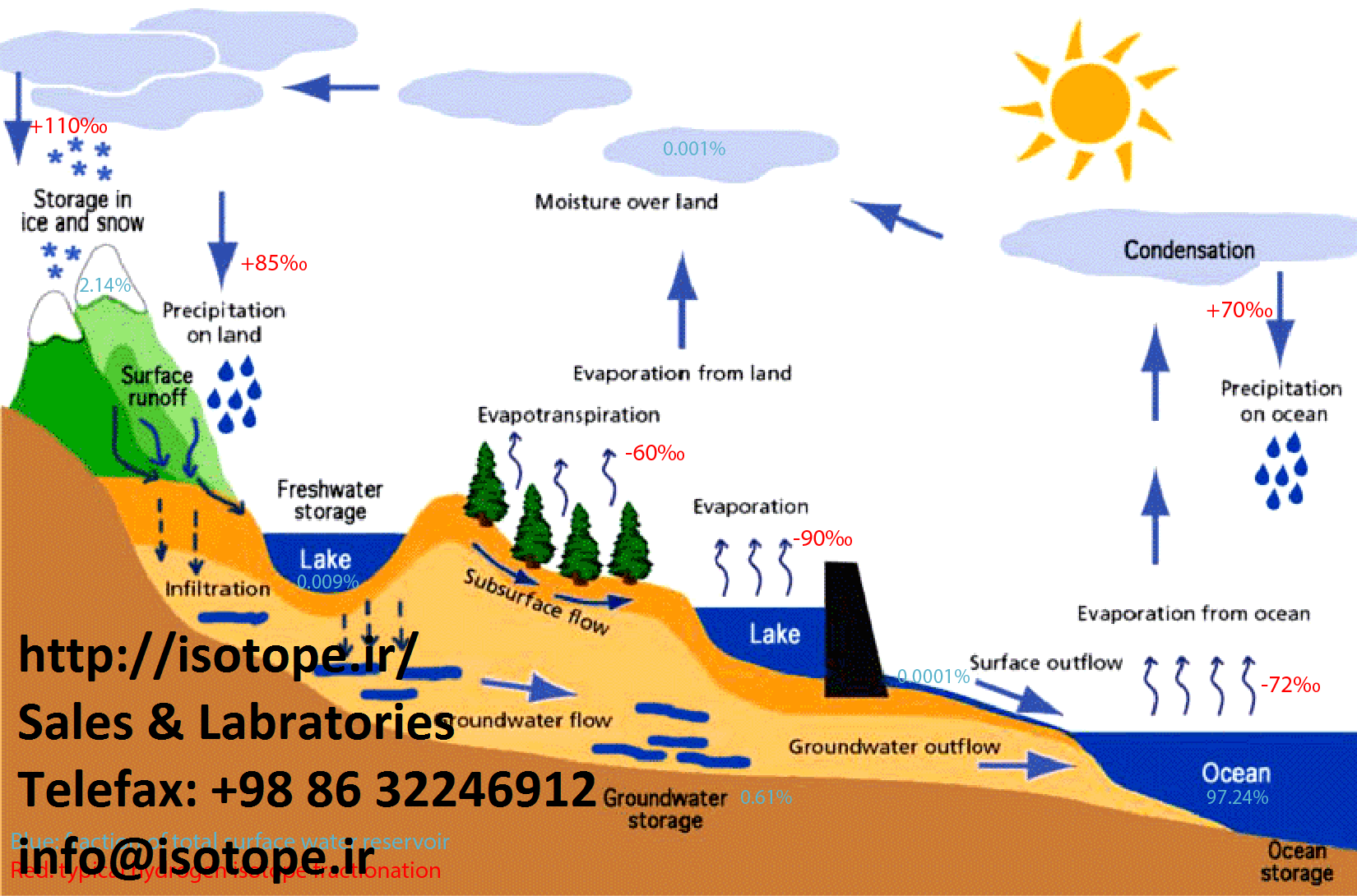
Isotopes, when used in groundwater hydrology give a direct insight into the movement and distribution processes within aquifers. Techniques used in hydrology may be classified into three groups: environmental isotopes, artificial isotopes, and application of sealed radioactive sources. Isotope hydrology provides complementary information on the type, origin and age of groundwater. If the isotope content does not change within the aquifer, it will reflect the origin of the water, particularly the location, period and processes of recharge. If the isotope content changes along groundwater paths, this will reflect the history of the water, particularly the mixing, salinization and discharge processes.
The isotopes commonly employed in groundwater investigations are the heavy stable isotopes of the water molecule, deuterium and oxygen-18 and the radioactive isotopes, tritium and carbon-14.
As there are no harmful chemicals associated with deuterium oxide and deuterium oxide is a stable isotope, it’s hydrology application cause no environmental hazards.
References:
1. J.L. Terwey, 1984, Isotopes in groundwater hydrology.
2. R. Letolle, Ph.Olive, 2003, A short history of isotopes in hydrology.