The use of kinetic isotope effects in interpretation of organic reaction mechanisms has undergone a remarkable development. Nowadays, Kinetic isotope effect is considered to be one of the most essential and sensitive tools for the study of reaction mechanisms.
Kinetic isotope effect (KIE) refers to the change in the rate of a chemical reaction upon substitution of an atom in the reactants with one of its isotopes.
Primary kinetic isotope effect: may be found when a bond to the isotopically labeled atom is being formed or broken. The rate of a reaction involving a C–H bond is typically 6–10 times faster than the corresponding C–D bond.
Secondary kinetic isotope: This type of isotope effect is significant when the atom to which the isotopic atom is bonded undergoes a hybridization change. Which, change in the hybridization during the formation of the transition state would lead to considerable KIE on the corresponding reaction rate.
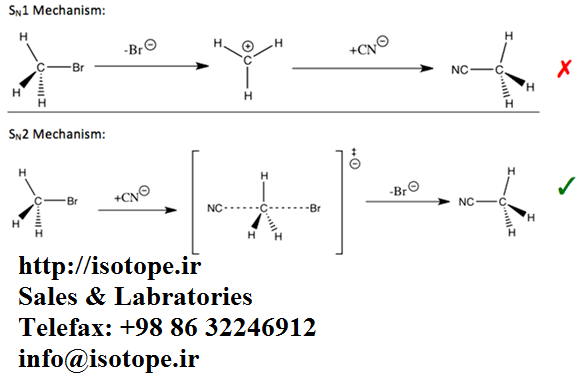
For example, kinetic isotope effects can be used to reveal whether a nucleophilic substitution reaction follows a unimolecular (SN1) or bimolecular (SN2) pathway.
1. U. Maitra, J. Chandrasekhar, Use of Isotopes for Studying Reaction Mechanisms, Secondary Kinetic Isotope Effect, Department of Organic Chemistry, Indian Institute of Science, Bangalore 560 012.
2. G. Hong, Sh. Zhang, Advances in Kinetic Isotope Effect Measurement Techniques for Enzyme Mechanism Study, Molecules, 2013, Vol. 18, 9278-9292.
3. P. J. SMITH, A. N. BOURNS, Isotope effect studies on elimination reactions, The mechanism of the bimolecular elimination reaction of arylethylammonium ions, CANADIAN JOURNAL OF CHEMISTRY, Vol. 48. 1970.