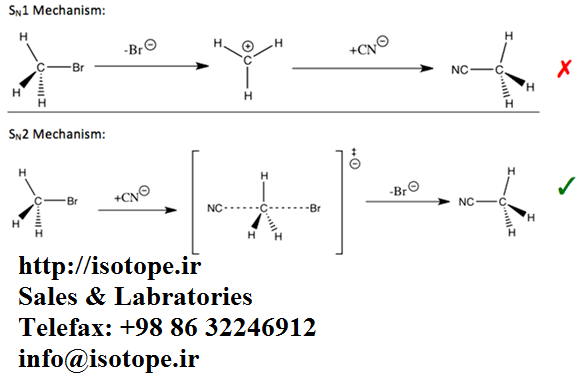
The breaking of carbon-hydrogen bonds is a common features of drug metabolism, for example during oxidation. Breaking of an analogues carbon-deuterium bond can be more difficult, and so decrease the rate of metabolism. Therefore, replacement of hydrogen with deuterium in drug molecules can lead to significant alterations in this metabolism and thereby cause beneficial changes in the biological effects of drugs, such as their pharmacokinetics by decreasing their rate of metabolism allowing less frequent dosing.
Deuterium labeling has effect of lowering toxicity by reducing the formation of a toxic metabolite.
References:>
1. S. L. Harbeson, R. D. Tung, Deuterium medicinal chemistry: A new approach to drug discovery and development, MEDCHEM NEWS No. 2, 2014.
2. G. S. Timins, Deuterated drugs; where are we now?, Expert opin ther pat., 2014